
Chemistry, 03.12.2019 20:31, Unkn0wn3815
At 700 k, kp for the following equilibrium is (5.6 x 10-3) 2hgo(> 2hg(l) + o2(g) suppose 51.2 g of mercury(ii) oxide is placed in a sealed 3.00-l vessel at 700 k. what is the partial
pressure of oxygen gas at equilibrium? (r = 0.0821 lxatm/(kxmol))
a) 0.075 atm
b) 0.0056 atm
c) 4.5 atm
d) 19 atm
e) 2.3 atm

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2


Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
Do you know the correct answer?
At 700 k, kp for the following equilibrium is (5.6 x 10-3) 2hgo(> 2hg(l) + o2(g) suppose 51.2 g...
Questions in other subjects:

History, 10.12.2020 23:00


Mathematics, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00


English, 10.12.2020 23:00


Mathematics, 10.12.2020 23:00

Physics, 10.12.2020 23:00

Mathematics, 10.12.2020 23:10


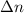 is as follows.
is as follows.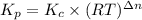
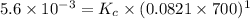
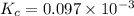
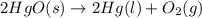
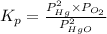
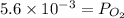
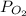 = 0.0056 atm
= 0.0056 atm



