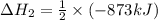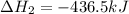The standard enthalpy change for the following reaction is 873 kj at 298 k.
2 kcl(s) 2 k(s) +...


Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, kelyanthecrafte
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1

Chemistry, 23.06.2019 13:00, nellys2096
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 23.10.2020 19:40


Biology, 23.10.2020 19:40



Biology, 23.10.2020 19:40

Mathematics, 23.10.2020 19:40




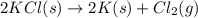
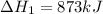
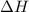 for the following reaction i.e,
for the following reaction i.e,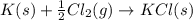
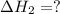
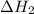 for the reaction will be:
for the reaction will be: