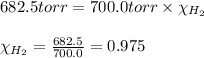Chemistry, 03.12.2019 18:31, taridunkley724
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of 700.0 torr. the partial pressure of water vapor at 20.00∘c is 17.5 torr. calculate the mole fraction of h2 gas in the sample.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
Do you know the correct answer?
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of...
Questions in other subjects:

Mathematics, 19.03.2020 23:34

Mathematics, 19.03.2020 23:34



English, 19.03.2020 23:34

History, 19.03.2020 23:34



Mathematics, 19.03.2020 23:34

English, 19.03.2020 23:34


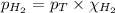
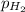 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr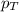 = total pressure = 700.0 torr
= total pressure = 700.0 torr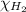 = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?