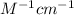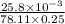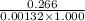Pure hexane has negligible ultraviolet absorbance above a wavelength of 200 nm. a solution prepared by dissolving 25.8 mg of benzene (c6h6, fm 78.11) in hexane and diluting to 250.0 ml had an absorption peak at 256 nm with an absorbance of 0.266 in a 1.000-cm cell. find the molar absorptivity of benzene at this wavelength.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
Do you know the correct answer?
Pure hexane has negligible ultraviolet absorbance above a wavelength of 200 nm. a solution prepared...
Questions in other subjects:




Arts, 26.03.2021 22:50

Business, 26.03.2021 22:50

Chemistry, 26.03.2021 22:50


Mathematics, 26.03.2021 22:50


Mathematics, 26.03.2021 22:50