
Chemistry, 03.12.2019 06:31, Theacefamily123
The following reaction is exothermic.
2 s(s) + 3 o2(g) → 2 so3(g)
what can we say about the spontaneity of this reaction?
(a) spontaneous at all temperatures
(b) spontaneous only at high temperatures
(c) spontaneous only at low temperatures
(d) non spontaneous at all temperatures
(e) more information is need to predict if the reaction is spontaneous

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 02:30, fordkenae
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Do you know the correct answer?
The following reaction is exothermic.
2 s(s) + 3 o2(g) → 2 so3(g)
what can...
2 s(s) + 3 o2(g) → 2 so3(g)
what can...
Questions in other subjects:

Mathematics, 14.11.2019 23:31






English, 14.11.2019 23:31


Geography, 14.11.2019 23:31


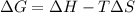
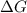 = Gibbs free energy
= Gibbs free energy 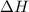 = enthalpy change
= enthalpy change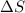 = entropy change
= entropy change 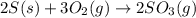
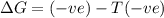
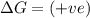 (at high temperature) (non-spontaneous)
(at high temperature) (non-spontaneous)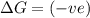 (at low temperature) (spontaneous)
(at low temperature) (spontaneous)



