
Chemistry, 03.12.2019 05:31, ErrorNameTaken505
The reaction of 50 ml of gas with 50 ml of gas via the equation: cl2(g) + c2h4(g) ➔ c2h4cl2 (g) will produce a total of ml of products if pressure and temperature are kept constant.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
Do you know the correct answer?
The reaction of 50 ml of gas with 50 ml of gas via the equation: cl2(g) + c2h4(g) ➔ c2h4cl2 (g) wil...
Questions in other subjects:











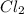 , 50 ml of
, 50 ml of 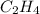
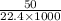 (As 1 L = 1000 ml)
(As 1 L = 1000 ml)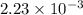 moles
moles 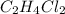 is equal to the moles of
is equal to the moles of 




