
Chemistry, 03.12.2019 04:31, applereams
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxygen (o2).
4al + 3o2 > 2al2o3
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 must react to form 3.80 mol of al2o3?
60.8
81.1
122
182

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, Mordred9571
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1


Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxy...
Questions in other subjects:

Mathematics, 11.06.2020 05:57


Law, 11.06.2020 05:57


History, 11.06.2020 05:57


Business, 11.06.2020 05:57



History, 11.06.2020 05:57


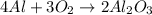
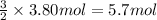 of oxygen gas.
of oxygen gas.



