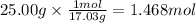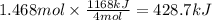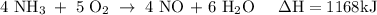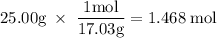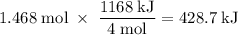Chemistry, 03.12.2019 04:31, averylivinglife2041
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to produce no(g) and h2o(l) according to the following chemical equation? 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(l) δh° = 1168 kj

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to...
Questions in other subjects:

Mathematics, 03.12.2019 19:31

History, 03.12.2019 19:31