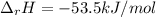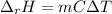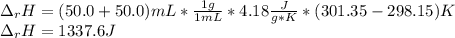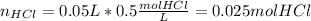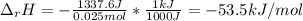Chemistry, 03.12.2019 04:31, dhananjaynagarkar
When 50.0 ml of 0.500 m hcl at 25.0°c is added to 50.0 ml of 0.500 m naoh at 25.0°c in a coffee cup calorimeter, the temperature of the mixture rises to 28.2°c.
what is the enthalpy of reaction per mole of acid?
assume the mixture has a specific heat capacity of 4.18 j/(g ? k) and that the densities of the reactant solutions are both 1.00 g/ml.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Do you know the correct answer?
When 50.0 ml of 0.500 m hcl at 25.0°c is added to 50.0 ml of 0.500 m naoh at 25.0°c in a coffee cup...
Questions in other subjects:

Mathematics, 06.05.2020 18:06

Business, 06.05.2020 18:06


Health, 06.05.2020 18:06

Mathematics, 06.05.2020 18:06

Mathematics, 06.05.2020 18:06




Mathematics, 06.05.2020 18:06