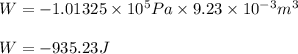Chemistry, 03.12.2019 04:31, jasmine2919
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 nan 3 ( s ) ⟶ 2 na ( s ) + 3 n 2 ( g ) calculate the value of work, w , for the system if 16.5 g nan 3 reacts completely at 1.00 atm and 22 ∘ c.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 07:30, jonquil201
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
Do you know the correct answer?
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heat...
Questions in other subjects:

Social Studies, 23.10.2020 19:40

Mathematics, 23.10.2020 19:40

Chemistry, 23.10.2020 19:40

Mathematics, 23.10.2020 19:40

Mathematics, 23.10.2020 19:40

Mathematics, 23.10.2020 19:40



Mathematics, 23.10.2020 19:40


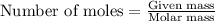
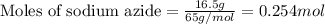
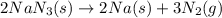
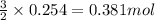 of nitrogen gas
of nitrogen gas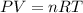
![22^oC=[22+273]K=295K](/tpl/images/0400/4731/7919f.png)
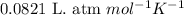
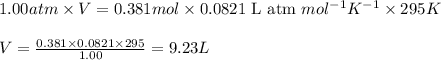
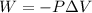
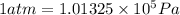 (Conversion factor: 1 atm = 101325 Pa)
(Conversion factor: 1 atm = 101325 Pa)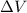 = change in volume =
= change in volume = 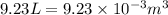 (Conversion factor:
(Conversion factor: 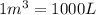 )
)