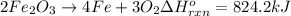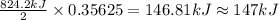Chemistry, 03.12.2019 03:31, dashavasilisk
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe + 3o2 deltah degree rxn = + 824.2 kj decomposition of 57.0 g of fe2o3 results in the release of 294 kj of heat. a. the absorption of 23500 kj of heat. b. the absorption of 147 kj of heat. c. the absorption of 294 kj of heat. d. the release of 23500 kj of heat. e. the release of 147 kj of heat.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
Do you know the correct answer?
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe +...
Questions in other subjects:

Mathematics, 18.02.2021 20:00


Medicine, 18.02.2021 20:00


Mathematics, 18.02.2021 20:00

Biology, 18.02.2021 20:00


Mathematics, 18.02.2021 20:00