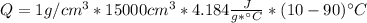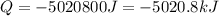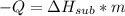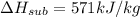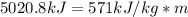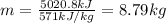Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the following equation: co2(s)→co2(g). when dry ice is added to warm water, heat from the water causes the dry ice to sublime more quickly. the evaporating carbon dioxide produces a dense fog often used to create special effects. in a simple dry ice fog machine, dry ice is added to warm water in a styrofoam cooler. the dry ice produces fog until it evaporates away, or until the water gets too cold to sublime the dry ice quickly enough. suppose that a small styrofoam cooler holds 15.0 liters of water heated to 90 ∘c. calculate the mass of dry ice that should be added to the water so that the dry ice completely sublimes away when the water reaches 10 ∘c. assume no heat loss to the surroundings.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
Do you know the correct answer?
Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the...
Questions in other subjects:




Geography, 12.07.2019 14:50

Geography, 12.07.2019 14:50

Geography, 12.07.2019 14:50

Mathematics, 12.07.2019 14:50


Social Studies, 12.07.2019 14:50

Biology, 12.07.2019 14:50


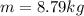
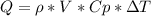
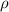 is the density
is the density