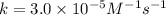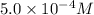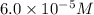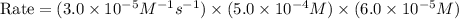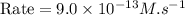Chemistry, 03.12.2019 01:31, PONBallfordM89
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has the following rate law at 310k.
rate of reaction=k[o3][no] k=3.0*10^6m^-1*s^-1
given that [o3]=5.0x10^-4m and no=6.0x10^-5m at t=0 calculate the rate of the reaction at t=0
what is the overall order of this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, lorenaandreahjimenez
The answer for #3 is c but i don't know why
Answers: 1

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Do you know the correct answer?
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has th...
o3(g)+no(> o2(g)+no2(g)
has th...
Questions in other subjects:



Physics, 28.09.2019 13:50


Computers and Technology, 28.09.2019 13:50

Mathematics, 28.09.2019 13:50

Business, 28.09.2019 13:50




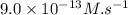
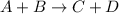
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0400/1843/10aeb.png)
![[A]](/tpl/images/0400/1843/6aa06.png) and
and ![[B]](/tpl/images/0400/1843/db909.png) = concentration of A and B reactant
= concentration of A and B reactant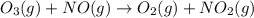
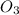 and
and 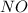 are the reactants.
are the reactants.![\text{Rate}=k[O_2][NO]](/tpl/images/0400/1843/1fdbe.png) ..........(1)
..........(1)