
Chemistry, 02.12.2019 23:31, tmrodriguez1
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of propane, and the standard enthalpy of combustion of gaseous propylene (c3h6) is –2058.3 kj per mole of propylene.
what is the standard enthalpy change for the following reaction at 25°c? c3h6(g) + h2(g) → c3h8(g)substance∆h°f (kj/mol)co2(g)–393.5h2o(l)–285.8

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
Do you know the correct answer?
At 25°c, the standard enthalpy of combustion of gaseous propane (c3h8) is –2219.0 kj per mole of pro...
Questions in other subjects:

Mathematics, 02.01.2021 22:00

Chemistry, 02.01.2021 22:00


Mathematics, 02.01.2021 22:00

Mathematics, 02.01.2021 22:00



Mathematics, 02.01.2021 22:00


History, 02.01.2021 22:00


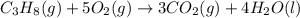
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/6eb42.png)
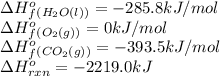
![-2219.0=[(3\times (-393.5))+(4\times (-285.8))]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times (0))]\\\\\Delta H^o_f_{(C_3H_8(g))}=-140.7kJ/mol](/tpl/images/0399/9365/987c0.png)
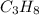 is -140.7 kJ/mol
is -140.7 kJ/mol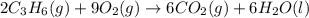
![\Delta H^o_{rxn}=[(6\times \Delta H^o_f_{(CO_2(g))})+(6\times \Delta H^o_f_{(H_2O(g))})]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0399/9365/b6201.png)
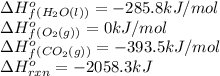
![-2058.3=[(6\times (-393.5))+(6\times (-285.8))]-[(2\times \Delta H^o_f_{(C_3H_6(g))})+(9\times (0))]\\\\\Delta H^o_f_{(C_3H_6(g))}=-1008.75kJ/mol](/tpl/images/0399/9365/ecedb.png)
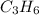 is -1008.75 kJ/mol
is -1008.75 kJ/mol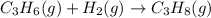
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(C_3H_8(g))})]-[(1\times \Delta H^o_f_{(C_3H_6(g))})+(1\times \Delta H^o_f_{(H_2(g))})]](/tpl/images/0399/9365/9b762.png)
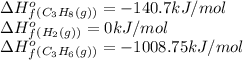
![\Delta H^o_{rxn}=[(1\times (-140.7))]-[(1\times (-1008.75))+(1\times (0))]\\\\\Delta H^o_{rxn}=868.05kJ](/tpl/images/0399/9365/ff366.png)




