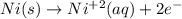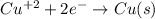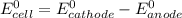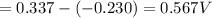Chemistry, 02.12.2019 21:31, loveashley1
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to their respective bins. view available hint(s) nickel copper standard reduction potentials for nickel(ii) and copper(ii) the standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. the more positive the reduction potential, the more easily the substance gains electrons. consider the following: ni2+(aq)+2e−→ni(s),cu2+(aq)+2e−→cu( s), e∘red=−0.230 v e∘red=+0.337 v part b what is the standard potential, e∘cell, for this galvanic cell? use the given standard reduction potentials in your calculation as appropriate.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, fireyking19
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal. a.added more engine powerb. activated a connection to the pedalc. stalled the engined. released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
Do you know the correct answer?
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to th...
Questions in other subjects:

Mathematics, 13.04.2021 16:00




Computers and Technology, 13.04.2021 16:00

Mathematics, 13.04.2021 16:00


Social Studies, 13.04.2021 16:00



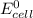 of the reaction is 0.567V.
of the reaction is 0.567V.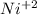 solution.
solution. 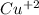 solution.
solution.