
Chemistry, 02.12.2019 21:31, krystalhurst97
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change for the following reaction.
(note: show the math clearly and provide units in your set up) ( hf values in kj/mol are as follows: no2 32, h2o 286, hno3 207, no 90.)
3no2(g) h2o(l) 2hno3(aq) no(g) g

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
Do you know the correct answer?
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change...
Questions in other subjects:

Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

English, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50

Business, 05.05.2020 05:50

Chemistry, 05.05.2020 05:50

Physics, 05.05.2020 05:50

Mathematics, 05.05.2020 05:50


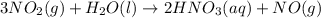
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0399/7869/76c37.png)
![\Delta H=[(n_{HNO_3}\times \Delta H_{HNO_3})+(n_{NO}\times \Delta H_{NO})]-[(n_{H_2O}\times \Delta H_{H_2O})+(n_{NO_2}\times \Delta H_{NO_2})]](/tpl/images/0399/7869/7081c.png)
![\Delta H=[(2\times -207)+(1\times 90)]-[(1\times -286)+(3\times 32)]](/tpl/images/0399/7869/1d6ad.png)





