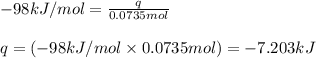Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
Do you know the correct answer?
Hydrogen peroxide decomposes to water and oxygen at constant pressure by the following reaction: 2h...
Questions in other subjects:

Chemistry, 27.09.2020 15:01



Mathematics, 27.09.2020 15:01



Mathematics, 27.09.2020 15:01




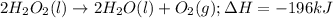
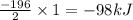
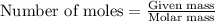
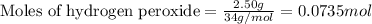
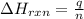
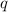 = amount of heat released
= amount of heat released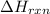 = enthalpy change of the reaction = -98 kJ/mol
= enthalpy change of the reaction = -98 kJ/mol