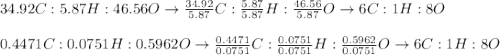Chemistry, 02.12.2019 15:31, ryleerose255
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. another sample is found to have 0.4471 g of carbon, 0.07510 g of hydrogen, and 0.5962 g of oxygen. show that these results are consistent with the law of definite proportions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
Do you know the correct answer?
Asample of glucose is found to have 34.92 g of carbon, 5.87 g of hydrogen and 46.56 g of oxygen. ano...
Questions in other subjects:

Mathematics, 09.04.2021 04:50

Mathematics, 09.04.2021 04:50


Physics, 09.04.2021 04:50


English, 09.04.2021 04:50

Mathematics, 09.04.2021 04:50

English, 09.04.2021 04:50

Mathematics, 09.04.2021 04:50