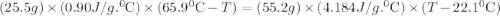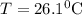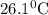Chemistry, 30.11.2019 07:31, Sylnatius6736
A25.5 −g aluminum block is warmed to 65.9 ∘c and plunged into an insulated beaker containing 55.2 g water initially at 22.1 ∘c. the aluminum and the water are allowed to come to thermal equilibrium.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
Do you know the correct answer?
A25.5 −g aluminum block is warmed to 65.9 ∘c and plunged into an insulated beaker containing 55.2 g...
Questions in other subjects:




Mathematics, 24.07.2019 05:30

Mathematics, 24.07.2019 05:30

Mathematics, 24.07.2019 05:30


Spanish, 24.07.2019 05:30

History, 24.07.2019 05:30

Health, 24.07.2019 05:30


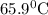 and plunged into an insulated beaker containing 55.2 g water initially at 22.1^{0}\textrm{C}. The aluminum and the water are allowed to come to thermal equilibrium. Assuming that no heat is lost, what is the final temperature of the water and aluminum?"
and plunged into an insulated beaker containing 55.2 g water initially at 22.1^{0}\textrm{C}. The aluminum and the water are allowed to come to thermal equilibrium. Assuming that no heat is lost, what is the final temperature of the water and aluminum?"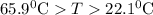
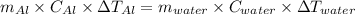
 represents change in temperature
represents change in temperature