
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge of its unit cell is 4.52 x 10-8cm.
a) how many atoms are in each unit cell?
b) what is the volume of a unit cell?
c) what is the mass of a unit cell?
d) calculate the approximate atomic mass of the element.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 23.06.2019 06:10, jamesgotqui6
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a. what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
Do you know the correct answer?
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge...
Questions in other subjects:





Mathematics, 30.07.2019 01:40

Mathematics, 30.07.2019 01:40

Social Studies, 30.07.2019 01:40



Health, 30.07.2019 01:40


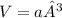 , a being the edge length.
, a being the edge length.



