
Chemistry, 30.11.2019 06:31, Virnalis1112
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and 0.42 m c6h5coona? (ka of benzoic acid = 6.3 × 10^−5). be sure to report your answer to the correct number of significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and...
Questions in other subjects:








History, 04.04.2020 06:07

Mathematics, 04.04.2020 06:07


![[H_{3}O^{+}]=x M = 2.5\times 10^{-5}M](/tpl/images/0397/1268/ab31e.png) and pH = 4.6
and pH = 4.6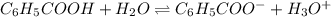
![\frac{[C_{6}H_{5}COO^{-}][H_{3}O^{+}]}{[C_{6}H_{5}COOH]}=K_{a}(C_{6}H_{5}COOH)](/tpl/images/0397/1268/75106.png)
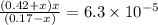
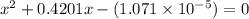
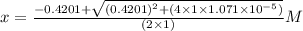
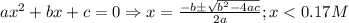 )
)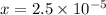 M
M![pH=-log[H_{3}O^{+}]=-logx=-log(2.5\times 10^{-5})=4.6](/tpl/images/0397/1268/f0ff1.png)




