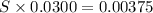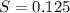What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.300 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq).

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
Do you know the correct answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions in other subjects:

Mathematics, 18.05.2021 22:20

History, 18.05.2021 22:20

Health, 18.05.2021 22:20

Mathematics, 18.05.2021 22:20





Mathematics, 18.05.2021 22:20


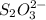 completely react with 1 mol of
completely react with 1 mol of 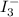 .
.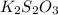 solution =
solution = 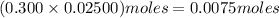
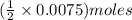 of
of 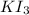 solution is S (M) then-
solution is S (M) then-