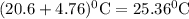Chemistry, 30.11.2019 05:31, kittykat7315
The δhcomb value for anethole is -5539 kj/mol. assume 0.840 g of anethole is combusted in a calorimeter whose heat capacity (calorimeter) is 6.60 kj/°c at 20.6 °c. what is the final temperature of the calorimeter

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
Do you know the correct answer?
The δhcomb value for anethole is -5539 kj/mol. assume 0.840 g of anethole is combusted in a calorime...
Questions in other subjects:











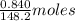 of anethole = 0.00567 moles of anethole
of anethole = 0.00567 moles of anethole of heat or 31.41 kJ of heat
of heat or 31.41 kJ of heat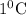 temperature of calorimeter.
temperature of calorimeter. or
or 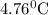 temperature of calorimeter
temperature of calorimeter