
Chemistry, 30.11.2019 05:31, rubyhart522
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, including bottled water. a solution is made by adding 6.50 g of cacl2 to 60.0 ml of water at 25∘c. the density of the solvent at that temperature is 0.997 g/ml. calculate the mole percent of cacl2 in the solution.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
Do you know the correct answer?
Calcium chloride, cacl2, is commonly used as an electrolyte in sports drinks and other beverages, in...
Questions in other subjects:






Mathematics, 01.12.2020 21:10




Mathematics, 01.12.2020 21:10


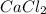 in solution is 1.71%
in solution is 1.71%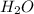 = 18.02 g/mol
= 18.02 g/mol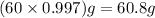
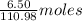 of
of 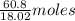 of
of 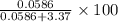 % = 1.71%
% = 1.71%



