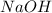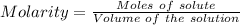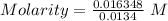Chemistry, 30.11.2019 05:31, brockandersin
The dissolution of 0.200 l of sulfur dioxide at 19 °c and 745 mmhg in water yields 500.0 ml of aqueous sulfurous acid. the solution is titrated with 13.4 ml of sodium hydroxide. what is the molarity of naoh?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
Do you know the correct answer?
The dissolution of 0.200 l of sulfur dioxide at 19 °c and 745 mmhg in water yields 500.0 ml of aqueo...
Questions in other subjects:


Mathematics, 08.11.2020 04:30

Mathematics, 08.11.2020 04:30

Spanish, 08.11.2020 04:30

English, 08.11.2020 04:30

English, 08.11.2020 04:30

Mathematics, 08.11.2020 04:30

Computers and Technology, 08.11.2020 04:30

English, 08.11.2020 04:30

Biology, 08.11.2020 04:30


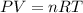
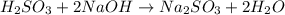
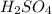 react with 2 moles of
react with 2 moles of