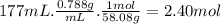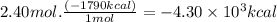Chemistry, 30.11.2019 05:31, gslovestodance6879
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredient innail polish remover. c3h6o(l) + 4o2 (g) > 3co2 (g) + 3h2o (g), δhoof the reaction = -1790 kcalif a bottle of nail polish remover contains 177 ml of acetone, how much heat would be released by its complete combustion? thedensity of acetone is 0.788 g/ml.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2
Do you know the correct answer?
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredi...
Questions in other subjects:

Mathematics, 24.06.2019 06:00


Mathematics, 24.06.2019 06:00

Mathematics, 24.06.2019 06:00


Mathematics, 24.06.2019 06:00

History, 24.06.2019 06:00



English, 24.06.2019 06:00