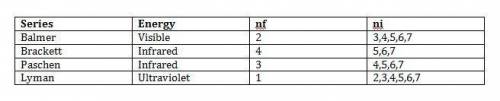
Chemistry, 30.11.2019 03:31, brooklyn674
The four sets of lines in the hydrogen emission spectrum are known as balmer, brackett, paschen, and lyman. for each series, assign the energy (infrared, ultraviolet, or visible), the nr value, and all possible n, values up to 7 series balmer brackett paschen lyman energy nf ni visible) infrared ultraviolet 1 2 3 6 7

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
Do you know the correct answer?
The four sets of lines in the hydrogen emission spectrum are known as balmer, brackett, paschen, and...
Questions in other subjects:







Physics, 20.09.2020 20:01

Social Studies, 20.09.2020 20:01

Mathematics, 20.09.2020 20:01

Mathematics, 20.09.2020 20:01