
Chemistry, 30.11.2019 03:31, emily200705
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of equilibrium for that reaction?
a. there are more reactants than products at equilibrium.
b. there are more products than reactants at equilibrium.
c. at equilibrium, the concentration of reactants is the same as that of the products.
d. the concentration of the product at equilibrium is 0.00010 m.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2


Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
Do you know the correct answer?
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of...
Questions in other subjects:

Health, 26.04.2021 09:30



Mathematics, 26.04.2021 09:30

Geography, 26.04.2021 09:30

Mathematics, 26.04.2021 09:30


Mathematics, 26.04.2021 09:30

Computers and Technology, 26.04.2021 09:30

Computers and Technology, 26.04.2021 09:30


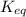
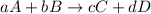
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0396/7601/9c8b0.png)
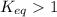 ; the reaction is product favored.
; the reaction is product favored.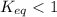 ; the reaction is reactant favored.
; the reaction is reactant favored.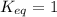 ; the reaction is in equilibrium.
; the reaction is in equilibrium.



