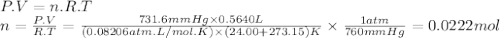Chemistry, 30.11.2019 03:31, fattypickeltoefungus
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experiment, a sample of kclo3 reacts and the gas produced is collected by water displacement. the gas sample has a temperature of 24.00 °c, a volume of 564.0 ml, and a pressure of 754.0 mm hg.
calculate the amount (in moles) of oxygen gas produced in the reaction. the vapor pressure of water is 22.38 mm hg at 24.00 °c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, aeverettpdzrvo
The most efficient way to establish the best possible economizer position is to measure
Answers: 1


Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
Do you know the correct answer?
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experime...
Questions in other subjects:




English, 30.12.2019 11:31

English, 30.12.2019 11:31


Mathematics, 30.12.2019 11:31

Business, 30.12.2019 11:31

Business, 30.12.2019 11:31