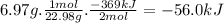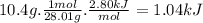The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium hydroxide(aq) and hydrogen(g). 2na(s) + 2h2o(l)2naoh(aq) + h2(g) h = -369 kj when 6.97 grams of sodium(s) react with excess water(l), kj of energy are .
2) the following thermochemical equation is for the reaction of carbon monoxide(g) with water(l) to form carbon dioxide(g) and hydrogen(g).
co(g) + h2o(l)arrow. gif co2(g) + h2(g) delta16-1.gifh = 2.80 kj
when 10.4 grams of carbon monoxide(g) react with excess water(l), kj of energy are .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, NetherisIsTheQueen
Apackage contains 1.33 lb of ground round. if there’s 29% fat, how many grams of fat are in the round? i got 175 g but the textbook says the answer is 91 g of fat. how?
Answers: 2



Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium...
Questions in other subjects:


Biology, 14.05.2021 16:50

Chemistry, 14.05.2021 16:50

Biology, 14.05.2021 16:50


Mathematics, 14.05.2021 16:50

Spanish, 14.05.2021 16:50

Mathematics, 14.05.2021 16:50


Mathematics, 14.05.2021 16:50