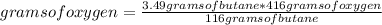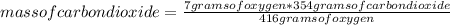Chemistry, 30.11.2019 03:31, fespinoza019
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. suppose 3.49 g of butane is mixed with 7.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
Do you know the correct answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. s...
Questions in other subjects:


Social Studies, 17.11.2019 15:31

Mathematics, 17.11.2019 15:31


Biology, 17.11.2019 15:31

English, 17.11.2019 15:31





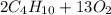 ⇒
⇒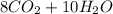
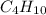 : 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol
: 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol