Chemistry, 30.11.2019 02:31, SapphireWolf
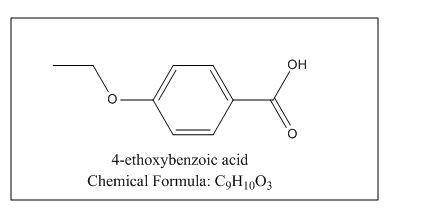
Provide a structure for the following compound: c9h10o3; ir: 2400–3200, 1700, 1630 cm–1; 1h nmr: δ 1.53 (3h, t, j = 8 hz); δ 4.32 (2h, q, j = 8 hz); δ 7.08, δ 8.13 (4h, pair of leaning doublets, j = 10 hz); δ 10 (1h, broad, disappears with d2o shake

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, sriggins1375
Which statement accurately describes the rock layers? layer 8 is older than layer 1. layer 3 is younger than layer 6. layer 4 and layer 10 are the same relative age. layer 2 and layer 9 are the same relative age.
Answers: 3


Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 08:00, katelyn0579
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely. analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1
Do you know the correct answer?
Provide a structure for the following compound: c9h10o3; ir: 2400–3200, 1700, 1630 cm–1; 1h nmr:...
Questions in other subjects:







English, 20.08.2020 17:01

Mathematics, 20.08.2020 17:01


Mathematics, 20.08.2020 17:01


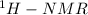 data,
data,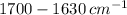 indicates the presence of carbonyl group.
indicates the presence of carbonyl group.