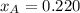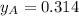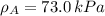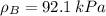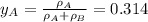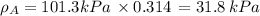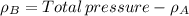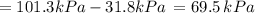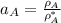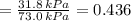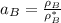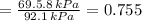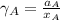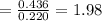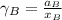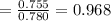By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it was found that xa = 0.220 when ya = 0.314. calculate the activities and activity coefficients of both components in this solution on the raoult’s law basis. the vapour pressures of the pure components at this temperature are: pa = 73.0 kpa and pb = 92.1 kpa. (xa is the mole fraction in the liquid and ya the mole fraction in the vapour.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, aeverettpdzrvo
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
Do you know the correct answer?
By measuring the equilibrium between liquid and vapour phases of a solution at 30°c at 1.00 atm, it...
Questions in other subjects:

Biology, 13.10.2019 03:20

Mathematics, 13.10.2019 03:20

Biology, 13.10.2019 03:20



History, 13.10.2019 03:20



English, 13.10.2019 03:20


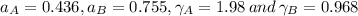 .
.