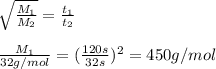Chemistry, 30.11.2019 01:31, hannahbeccahxo9681
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. it required 120 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 32 s for 1.0 l of o2 gas to effuse. you may want to reference (pages 416 - 419) section 10.8 while completing this problem. part a calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 l; that is, rate and time are inversely proportional.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1


Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
Do you know the correct answer?
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure...
Questions in other subjects:

Chemistry, 07.03.2020 02:27