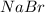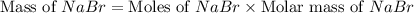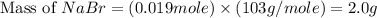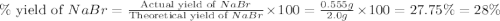Chemistry, 30.11.2019 01:31, Gigglygoose4181
Aqueous hydrobromic acid hbr reacts with solid sodium hydroxide naoh to produce aqueous sodium bromide nabr and liquid water h2o . if 0.555g of sodium bromide is produced from the reaction of of 2.4g hydrobromic acid and 0.77g of sodium hydroxide, calculate the percent yield of sodium bromide.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
Do you know the correct answer?
Aqueous hydrobromic acid hbr reacts with solid sodium hydroxide naoh to produce aqueous sodium bromi...
Questions in other subjects:





Health, 24.11.2021 17:10

Law, 24.11.2021 17:10





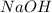
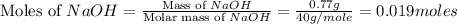
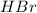
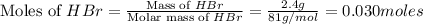
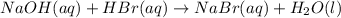
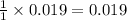 mole of
mole of