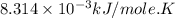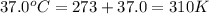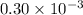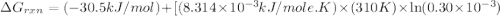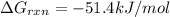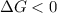Chemistry, 30.11.2019 01:31, redhot12352
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp(aq) +h2o (l) > adp(aq) +hpo4 (negative two overall charge) (aq).
for which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.30 mm, and [hpo4^2–] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3


Do you know the correct answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions in other subjects:

Mathematics, 17.11.2019 04:31


English, 17.11.2019 04:31

Health, 17.11.2019 04:31


Biology, 17.11.2019 04:31



History, 17.11.2019 04:31

Health, 17.11.2019 04:31


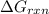 is -51.4 kJ/mol
is -51.4 kJ/mol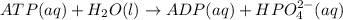
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0396/5636/ccdf0.png)
![[ATP]](/tpl/images/0396/5636/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0396/5636/68360.png) = 0.30 mM
= 0.30 mM![[HPO_4^{2-}]](/tpl/images/0396/5636/c0ca9.png) = 5.0 mM
= 5.0 mM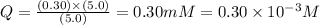
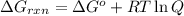 ............(1)
............(1)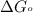 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol