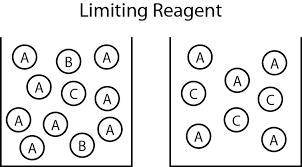
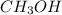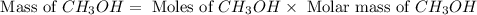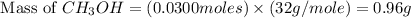Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2(g)→ch3oh(g) a 1.65 l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 374 mmhg .identify the limiting reactant and determine the theoretical yeild of methonal in grams.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
Do you know the correct answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2...
Questions in other subjects:











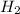 and the theoretical yield of methanol is, 0.96 grams.
and the theoretical yield of methanol is, 0.96 grams.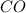 and
and 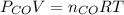
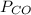 = pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)
= pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)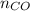 = number of moles of CO gas = ?
= number of moles of CO gas = ?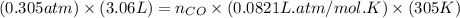
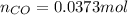
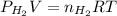
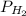 = pressure of
= pressure of 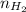 = number of moles of
= number of moles of 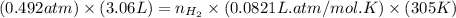
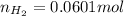
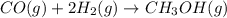
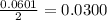 moles of
moles of