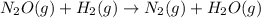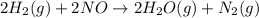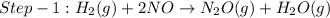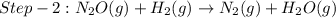Chemistry, 30.11.2019 01:31, uticabadgirl
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g) 2h20 ()n2 (g suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: h2 g+2no(g)- n20 (g)+h20(g) suppose also that the second step of the mechanism should be bimolecular suggest a reasonable second step. that is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1


Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g...
Questions in other subjects:

English, 10.03.2021 21:20

Mathematics, 10.03.2021 21:20



Mathematics, 10.03.2021 21:20


Mathematics, 10.03.2021 21:20


Mathematics, 10.03.2021 21:20

Mathematics, 10.03.2021 21:20