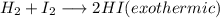Chemistry, 29.11.2019 07:31, heathkid23
 + i_2(g) \longrightarrow 2hi(g))
the forward reaction above is exothermic. at equilibrium, what happens if the reaction mixture is cooled at constant volume?
select all that apply.
1. the reaction absorbs energy.
2. the reaction releases energy.
3. [h₂] and [i₂] increase.
4. [h₂] and [i₂] decrease.
5. [h₂] and [i₂] remain constant.
6. [hi] increases.
7. [hi] decreases.
8. [hi] remains constant.
if c is added to the equilibrium system above, in which direction will the equilibrium shift?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3
Do you know the correct answer?
[tex]h_2(g) + i_2(g) \longrightarrow 2hi(g)[/tex]
the forward reaction above is exothermic. at...
the forward reaction above is exothermic. at...
Questions in other subjects:

Chemistry, 26.02.2021 17:50


Geography, 26.02.2021 17:50

Chemistry, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50





Mathematics, 26.02.2021 17:50


![[H_{2}]](/tpl/images/0395/9023/f6f78.png) and
and ![[I_{2}]](/tpl/images/0395/9023/d0626.png) increase.
increase.![[HI]](/tpl/images/0395/9023/7d1e5.png) increases.
increases.