Chemistry, 29.11.2019 06:31, cathydaves
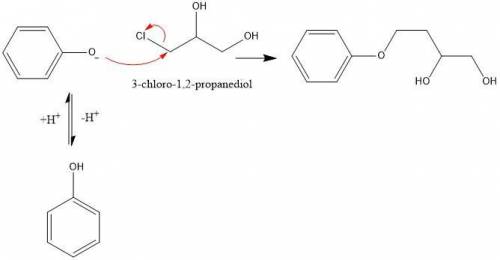
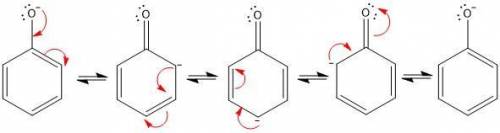
1. in the first step of the mechanism for this process, a phenoxide anion is generated. this phenoxide anion goes on to act as a nucleophile via an sn2 mechanism, displacing the chloride on 3-chloro-1,2-propanediol. why doesn’t the phenoxide anion act as a base to deprotonate one of the alcohols on 3-chloro-1,2-propanediol? write a brief, specific explanation (1-2 sentences).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 23.06.2019 11:00, artiomtyler007
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 11:00, jdisalle2808
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
Do you know the correct answer?
1. in the first step of the mechanism for this process, a phenoxide anion is generated. this phenoxi...
Questions in other subjects:

Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31


Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31

Biology, 11.12.2019 07:31

Social Studies, 11.12.2019 07:31



Spanish, 11.12.2019 07:31