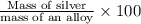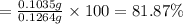Chemistry, 29.11.2019 05:31, SophieCasey
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silver precipitated as silver chloride, the precipitate had a mass of 0.1375g. calculate the percent of silver in the alloy. for full credit, make sure you show your calculations.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3


Do you know the correct answer?
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silv...
Questions in other subjects:


Geography, 29.10.2020 18:50




Health, 29.10.2020 18:50

Biology, 29.10.2020 18:50

History, 29.10.2020 18:50



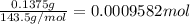
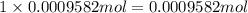 of silver
of silver