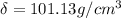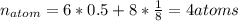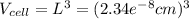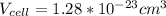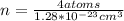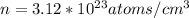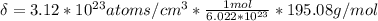Chemistry, 29.11.2019 05:31, ajayfurlow
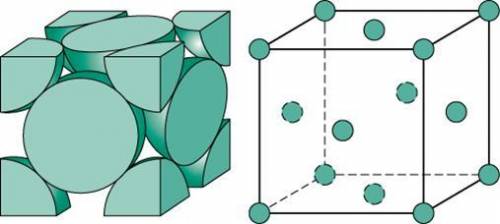
Ahypothetical metal crystallizes with the face-centered cubic unit cell. the radius of the metal atom is 234 picometers and its molar mass is 195.08 g/mol. calculate the density of the metal in g/cm3.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
Do you know the correct answer?
Ahypothetical metal crystallizes with the face-centered cubic unit cell. the radius of the metal ato...
Questions in other subjects:


Chemistry, 23.03.2021 21:30

Mathematics, 23.03.2021 21:30


History, 23.03.2021 21:30



English, 23.03.2021 21:30


Mathematics, 23.03.2021 21:30