
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation constant, a student dissolved 2.00 g of aspirin in 0.600 l of water and measured the ph. what was the ka value calculated by the student if theph of the solution was 2.62? a 0.100 m solution of ethylamine (c2h5nh2) has a ph of 11.87. calculate the kb for ethylamine.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, arnold2619
What is the force of attraction between the particles in a salt crystal
Answers: 2


Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
Do you know the correct answer?
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation...
Questions in other subjects:

Mathematics, 01.04.2021 16:50



Mathematics, 01.04.2021 16:50


Mathematics, 01.04.2021 16:50



Mathematics, 01.04.2021 16:50


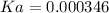

 = 180.16 g/mol
= 180.16 g/mol
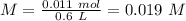
![Ka=\frac{[X][X]}{[0.019-X]}=\frac{[X]^2}{[0.019-X]}](/tpl/images/0395/6760/646b6.png)
![pH=-Log[H^+]](/tpl/images/0395/6760/3ca39.png)
![[H^+]=10^-^p^H](/tpl/images/0395/6760/16952.png)
![[H^+]=10^-^2^.^6^2=0.00240](/tpl/images/0395/6760/b62ea.png)
![Ka=\frac{[0.00240]^2}{[0.019-0.00240]}=0.000346](/tpl/images/0395/6760/6c4c9.png) .
.![Kb=\frac{[X][X]}{[0.1-X]}=\frac{[X]^2}{[0.1-X]}](/tpl/images/0395/6760/c1bde.png)


![pOH=-Log[OH^-]](/tpl/images/0395/6760/626fd.png)
![[OH^-]=10^-^p^O^H](/tpl/images/0395/6760/86a8e.png)
![[OH^-]=10^-^2^.^1^3=0.00741](/tpl/images/0395/6760/49ab9.png)
![Kb=\frac{[0.00741]^2}{[0.1-0.00741]}=0.000593](/tpl/images/0395/6760/bbeaa.png)




