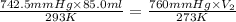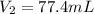Chemistry, 29.11.2019 02:31, Emptypockets451
If the volume of wet gas collected over water is 85.0 ml at 20°c and 760 mm hg , what is the volume of dry gas at stp conditions? (the vapor pressure of water at 20°c is 17.5 mm hg.) express your answer with the appropriate units.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, tristen2001
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3


Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
Do you know the correct answer?
If the volume of wet gas collected over water is 85.0 ml at 20°c and 760 mm hg , what is the volume...
Questions in other subjects:

Mathematics, 25.06.2021 22:30





Mathematics, 25.06.2021 22:30

Mathematics, 25.06.2021 22:30


English, 25.06.2021 22:30

Mathematics, 25.06.2021 22:30


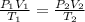
 = initial pressure of dry gas = (760 - 17.5) mmHg= 742.5 mm Hg
= initial pressure of dry gas = (760 - 17.5) mmHg= 742.5 mm Hg = final pressure of dry gas at STP = 760 mm Hg
= final pressure of dry gas at STP = 760 mm Hg = initial volume of dry gas = 85.0 mL
= initial volume of dry gas = 85.0 mL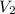 = final volume of dry gas at STP = ?
= final volume of dry gas at STP = ? = initial temperature of dry gas =
= initial temperature of dry gas = 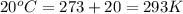
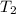 = final temperature of dry gas at STP =
= final temperature of dry gas at STP =