
Chemistry, 29.11.2019 02:31, Satoetoe24
Asample of impure tin of mass 0.526 g is dissolved in strong acid to give a solution of sn2 . the solution is then titrated with a 0.0448 m solution of no3−, which is reduced to no(g). the equivalence point is reached upon the addition of 3.67×10−2 l of the no3− solution.
find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
Do you know the correct answer?
Asample of impure tin of mass 0.526 g is dissolved in strong acid to give a solution of sn2 . the so...
Questions in other subjects:




Mathematics, 10.08.2021 21:20





Mathematics, 10.08.2021 21:20

Biology, 10.08.2021 21:20


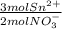 = 2.47x10⁻³mol Sn²⁺
= 2.47x10⁻³mol Sn²⁺



