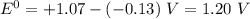Chemistry, 29.11.2019 00:31, savyblue1724707
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°c. (the equation is balanced.) pb(s) + br2(l) → pb2+(aq) + 2br(aq) pb2+(aq) + 2 e → pb(s) e° = -0.13 v br2(l) + 2 e → 2 br(aq) e° = +1.07 v

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1
Do you know the correct answer?
Use the standard half-cell potentials listed below to calculate the standard cell potential for the...
Questions in other subjects:

History, 11.10.2020 22:01

English, 11.10.2020 22:01

Biology, 11.10.2020 22:01



Spanish, 11.10.2020 22:01

Social Studies, 11.10.2020 22:01

Law, 11.10.2020 22:01

Mathematics, 11.10.2020 22:01


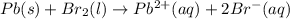
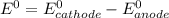
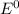 are standard reduction potentials.
are standard reduction potentials.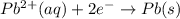
![E^0_{[Pb^{2+}/Pb]}= -0.13\ V](/tpl/images/0395/3322/82712.png)
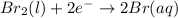
![E^0_{[Br_2/Br^{-}]}=+1.07\ V](/tpl/images/0395/3322/f8f7e.png)
![E^0=E^0_{[Br_2/Br^{-}]}- E^0_{[Pb^{2+}/Pb]}](/tpl/images/0395/3322/fba3a.png)