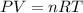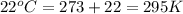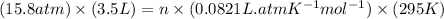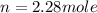Chemistry, 28.11.2019 21:31, carleenespriu
How many moles of gas must be forced into a 3.5 l ball to give it a gauge pressure of 8.8 psi at 22 ∘c? the gauge pressure is relative to atmospheric pressure. assume that atmospheric pressure is 14.5 psi so that the total pressure in the ball is 23.3 psi .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
Do you know the correct answer?
How many moles of gas must be forced into a 3.5 l ball to give it a gauge pressure of 8.8 psi at 22...
Questions in other subjects:


Biology, 13.02.2022 01:00

Health, 13.02.2022 01:00


Mathematics, 13.02.2022 01:00

Physics, 13.02.2022 01:00

Biology, 13.02.2022 01:00

Geography, 13.02.2022 01:00

Chemistry, 13.02.2022 01:00