If you are given an ideal gas with pressure (p) = 259,392.00 pa and temperature (t) = 2.00 oc
...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
Do you know the correct answer?
Questions in other subjects:

History, 05.03.2021 05:20

Physics, 05.03.2021 05:20

History, 05.03.2021 05:20




Mathematics, 05.03.2021 05:20

Mathematics, 05.03.2021 05:20


Mathematics, 05.03.2021 05:20


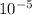 atm
atm



