
Chemistry, 28.11.2019 06:31, mxltie1651
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. round each of your answers to 3 significant digits. sulfur tetraflouride: mole fraction? partial pressure? carbon dioxide: mole fraction? partial pressure? total pressure in tank?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 03:00, andrejr0330jr
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 15:30, bm42400
The smallest species of chameleon is found only on an island in madagascar the island has unique geological and climatic conditions that are essential for the chamaeleon to survive the chamaeleon was recently declared an endangered species years from now why won’t this chameleons remains make for a good index fossil
Answers: 3
Do you know the correct answer?
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide...
Questions in other subjects:

Physics, 15.04.2021 03:10


English, 15.04.2021 03:10

Mathematics, 15.04.2021 03:10

Mathematics, 15.04.2021 03:10



Chemistry, 15.04.2021 03:10


Mathematics, 15.04.2021 03:10


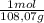 = 0,0489 moles SF₄
= 0,0489 moles SF₄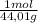 = 0,354 moles CO₂
= 0,354 moles CO₂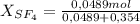 = 0,121
= 0,121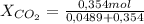 = 0,879
= 0,879



