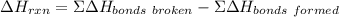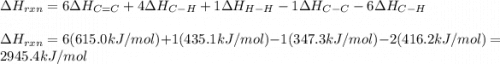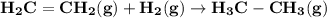Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1
Do you know the correct answer?
Use average bond energies to calculate δhrxn for the following hydrogenation reaction: h2c=ch2(g)+h...
Questions in other subjects:

Mathematics, 06.05.2020 00:01



Arts, 06.05.2020 00:01

Biology, 06.05.2020 00:01

Mathematics, 06.05.2020 00:01


Mathematics, 06.05.2020 00:01

Mathematics, 06.05.2020 00:01

Chemistry, 06.05.2020 00:01